Global Bio-pharmaceutical IPR Solutions
Tailored biopharmaceutical, medicolegal, and healthcare solutions for domestic and international clients.
Contact us for assistance.
We don’t just provide services; we build relationships. Our clients are our partners, and your success is our success. Thank you for placing your trust in us. We look forward to supporting you in achieving your goals and advancing innovation in the healthcare and legal domains.
IPR Services
Expert solutions in Biopharmaceutical, healthcare, and medicolegal consultancy.
CONNECTING THE DOTS..... WITH EXPERTS FROM DIFFERENT DOMAINS.
We understand the dynamic needs of our clients, whether they are startups forging a growth path or established enterprises safeguarding their assets. With a steadfast focus on quality and efficiency, we are here to ensure that your business operates with the highest levels of integrity and compliance.
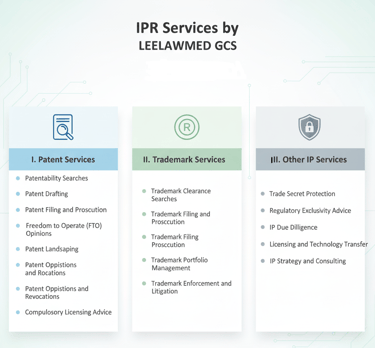
Patent Services:
Patentability Searches: Conducting thorough searches to determine the novelty and inventiveness of pharmaceutical inventions (new chemical entities, formulations, processes, etc.).
Patent Drafting: Preparing detailed and legally sound patent applications.
Patent Filing and Prosecution: Managing the patent application process with the Patent Office and other international patent offices.
Patent Portfolio Management: Assisting companies in strategically managing their patent assets.
Freedom-to-Operate (FTO) Opinions: Analyzing the patent landscape to assess the risk of infringing existing patents before launching a new product.
Patent Landscaping: Providing insights into the patent activity in specific therapeutic areas or technologies.
Patent Oppositions and Revocations: Representing clients in pre-grant and post-grant opposition proceedings and revocation petitions.
Compulsory Licensing Advice: Guiding companies on issues related to compulsory licensing of pharmaceutical patents.
Trademark Services:
Trademark Clearance Searches: Ensuring that brand names and logos are available for use and registration.
Trademark Filing and Prosecution: Registering trademarks for pharmaceutical products and company names.
Trademark Portfolio Management: Managing and protecting pharmaceutical trademarks.
Trademark Enforcement and Litigation: Handling cases of trademark infringement and passing off.
Trade Secret Protection:
Advising on strategies to protect confidential information, such as manufacturing processes, formulations, and clinical trial data.
Regulatory Exclusivity Advice:
Guiding companies on data exclusivity and other regulatory protections available for pharmaceutical products.
IP Due Diligence:
Conducting IP audits for mergers and acquisitions, licensing agreements, and other transactions in the pharmaceutical sector.
Licensing and Technology Transfer:
Drafting and negotiating licensing agreements for pharmaceutical patents, trademarks, and technologies.
Litigations guidelines:
Our litigation advisors provide strategic representation for clients in resolving disputes effectively. We guide and facilitate the required advocate for your interests, offering robust legal solutions in complex cases.Intellectual Property:
We specialize in guiding protecting and managing intellectual property rights across various industries. Our team helps to explore a potential way to secure trademarks, patents, copyrights, and trade secrets to maintain a competitive edge.Ethics, Risk, Compliance:
We help businesses navigate ethical challenges, manage risks, and maintain regulatory compliance. Our consultancy ensures that your company operates within legal and ethical boundaries.Paralegal:
Our paralegal services support international legal teams by handling research, document preparation, and case management. We offer skilled assistance to streamline legal processes and enhance efficiency.IP Strategy and Consulting:
Developing overall IPR strategies aligned with the business goals of pharmaceutical companies.
Advising on the commercialization of IP assets.

Securing Your Intellectual Property in the Medical Domain
With the growing need for original medical content, protecting intellectual property through copyright registration and licensing is essential. We offer services to help clients safeguard their work and monetize their intellectual property.
Our Services Include:
Copyright Registration & Protection – Assisting medical writers, researchers, and organizations in securing copyrights for their work.
Plagiarism Detection & Content Integrity Assurance – Ensuring originality and preventing unauthorized use through AI-assisted advanced plagiarism detection tools.
Content Licensing Agreements – Drafting and negotiating agreements for licensing medical content, ensuring fair compensation and legal protection.
Medical Content Distribution & Syndication – Helping clients legally distribute their content while maintaining ownership and control.
Intellectual Property (IP) Consultation – Offering expert advice on patentability, Trademarks, Brands, Trade Secrets, and Proprietary Research Protection.
By securing legal rights over medical content, we enable our clients to maximize the value of their intellectual assets while minimizing the risk of infringement.
Copyright Protection & Content Licensing
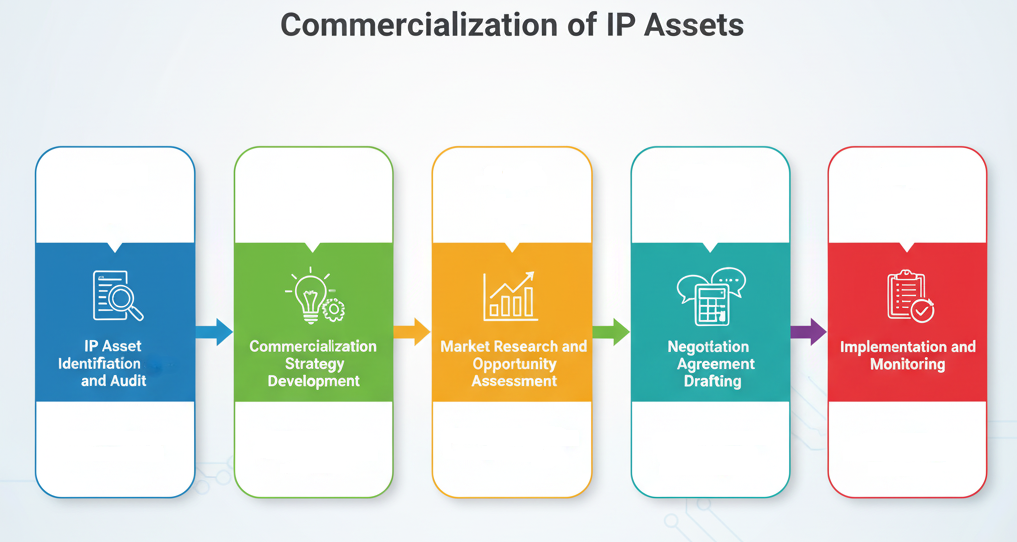
Advising on the commercialization of Intellectual Property (IP) assets is a multifaceted process that helps IP owners (like pharmaceutical companies) unlock the economic potential of their patents, trademarks, copyrights, and trade secrets. It goes beyond simply securing IP rights and focuses on how to leverage them to generate revenue, gain market share, or achieve strategic business objectives. Here's a breakdown of the key steps and considerations involved:
1. IP Asset Identification and Audit:
Comprehensive Review: The first step involves a thorough audit of the client's existing IP portfolio. This includes identifying all patents (granted and pending), trademarks (registered and applied for), copyrights, trade secrets, and any other forms of IP.
Valuation Assessment: A preliminary assessment of the potential commercial value of each IP asset is conducted. This considers factors like market demand, competitive landscape, technological relevance, and potential for exclusivity.
Strategic Alignment: Understanding the client's overall business strategy, goals, and resources is crucial to determine which IP assets are most relevant for commercialization efforts.
2. Commercialization Strategy Development:
Based on the IP audit and strategic alignment, various commercialization pathways are explored, and a tailored strategy is developed.
Common options include:
Direct Exploitation:
o Manufacturing and Selling: The IP owner directly manufactures and sells products or services protected by their IP. (e.g., a pharma company manufacturing and selling a patented drug).
o Service Provision: Using proprietary knowledge or processes (protected as trade secrets or patents) to offer unique services.
· Indirect Exploitation (Licensing):
o Exclusive Licensing: Granting one party the exclusive right to use, manufacture, and sell the IP in a specific territory or field of use.
o Non-Exclusive Licensing: Granting multiple parties the right to use the IP.
o Cross-Licensing: Exchanging IP rights with another party, often to enable product development or market access.
Sale or Assignment: Transferring ownership of the IP asset to another party for a lump-sum payment or ongoing royalties.
Strategic Partnerships and Collaborations:
o Joint Ventures: Forming a new entity with another company to jointly commercialize IP.
o Co-development Agreements: Collaborating with another party to further develop and commercialize IP.
Spin-offs: Creating a new company to focus on the commercialization of specific IP assets.
3.Market Research and Opportunity Assessment:
o Target Market Identification: Identifying the most promising markets and customer segments for the IP-protected products or services.
o Competitive Analysis: Analyzing the existing competitive landscape, including competing products, technologies, and IP.
o Market Demand Forecasting: Estimating the potential demand and revenue for the commercialized IP.
o Risk Assessment: Identifying potential risks associated with each commercialization strategy (e.g., infringement, market acceptance, regulatory hurdles).
4. Financial Modelling and Valuation:
o Cost-Benefit Analysis: Evaluating the potential costs and benefits associated with each commercialization option.
o Financial Projections: Developing financial forecasts, including revenue projections, cost estimates, and profitability analysis.
o IP Valuation: Employing various valuation methodologies (e.g., cost-based, market-based, income-based) to determine the fair market value of the IP asset for licensing or sale purposes.
5. Negotiation and Agreement Drafting:
o Identifying Potential Partners/Licensees/Buyers: Based on the chosen strategy and market research, identifying suitable parties for collaboration.
o Developing Negotiation Strategies: Preparing negotiation positions and tactics to achieve favorable terms.
o Drafting and Reviewing Agreements: Creating legally sound and commercially viable agreements (e.g., license agreements, sale agreements, collaboration agreements) that clearly define the rights, obligations, and financial terms.
6. Implementation and Monitoring:
o Supporting Implementation: Providing guidance and support during the initial stages of commercialization.
o Performance Monitoring: Establishing metrics to track the success of the commercialization strategy.
o Support Dispute Resolution: Advising on strategies to resolve any disputes that may arise during the commercialization process.
Commercialization of IP assets
Medicolegal Consultancy
Medicolegal consultancy involves experts with dual expertise in medicine and law, providing specialized guidance on regulatory frameworks affecting various healthcare sectors. This field focuses on advising hospitals, medical research institutes, long-term care facilities, nursing homes, clinics, pharmacies, pharmaceutical industries, clinical trial centers, contract research organizations (CROs), and other healthcare providers on legal compliance and risk management.
The legal regulations within this domain aim to ensure proper patient care while safeguarding their rights.
Health and Medicolegal Services
Key Stakeholders:
Healthcare Providers: Hospitals, doctors, nursing homes, and clinics
Pharmaceutical & Medical Industry: Pharmaceutical companies, medical device manufacturers, CROs
Regulatory & Government Bodies: Health authorities and policy-making agencies
Medicolegal consultants and healthcare attorneys play a crucial role in protecting patient rights, particularly in cases involving personal injury claims, financial settlements, and insurance disputes. Their responsibilities include interpreting and applying healthcare regulations to prevent legal violations and ensure compliance across various healthcare settings.
Key Areas of Medicolegal Consultancy
1. Healthcare Compliance & Risk Management
Medicolegal consultants advise healthcare professionals and organizations on regulatory compliance, helping them navigate complex healthcare laws and avoid unintentional breaches that may result in legal penalties.
2. Clinical Research Compliance & Ethical Standards
Ensuring adherence to clinical research regulations, including ethical considerations such as obtaining informed consent and complying with Good Clinical Practice (GCP) guidelines.
3. HIPAA & Data Protection in Healthcare
Consultants help healthcare providers understand their responsibilities under the Health Insurance Portability and Accountability Act (HIPAA), ensuring patient confidentiality and data protection.
L E E L A W M E D
GLOBAL CONSULTING SOLUTIONS
Lifeline of Science & Technologies
Expertise in Bio-Pharmaceutical Research, Medical Affairs, Medical Communication, Publication, MLR, Regulatory, IPRs, Medicolegal, and Healthcare Consulting Solutions.
Contact Us:
For more information
e-mail: info@leelawmed.com
Phone: +91-8722860211
© 2026 | www.leelawmed.com | All rights reserved.

