Global Healthcare and Biopharmaceutical
MLR Consulting Solutions
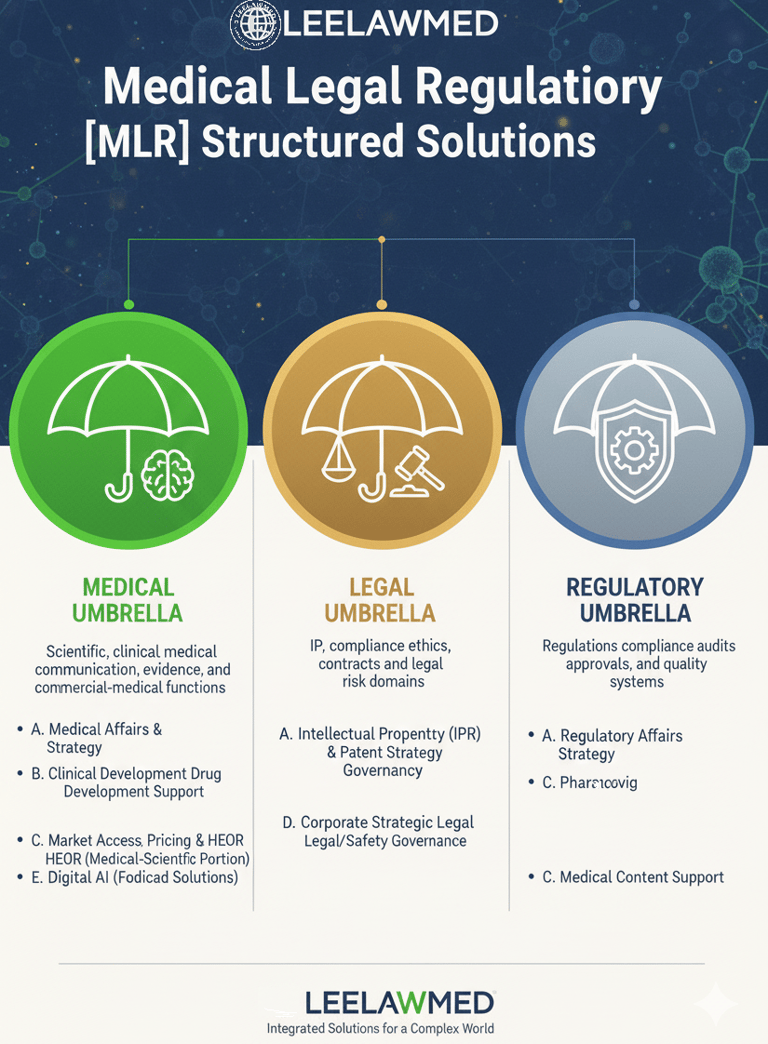
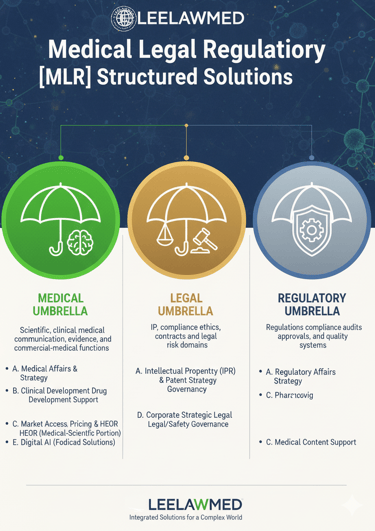
Leelawmed provides expert Medical-Legal-Regulatory (MLR) Structured Solutions tailored for discerning healthcare, biopharmaceutical, and MedTech clients, ensuring comprehensive support across critical operational domains.
Our MEDICAL UMBRELLA encompasses essential functions like Medical Affairs & Scientific Strategy, guiding clients through Clinical Development & Drug Development Support, and ensuring Medical Communication & Content Excellence. This pillar also manages the medical-scientific portions of Market Access, Pricing & HEOR, and leverages Digital & AI for medical-focused solutions.
Concurrently, the LEGAL UMBRELLA protects core assets through rigorous Intellectual Property (IPR) & Patent Strategy, establishes strong Compliance, Ethics & Legal Governance, offers Corporate & Strategic Legal Advisory, and addresses modern risks via Digital & AI for legal and safety governance.
Finally, the REGULATORY UMBRELLA secures market success with expert Regulatory Affairs & Strategy, meticulous Pharmacovigilance, and crucial Medical Content Support, ultimately delivering an integrated, risk-mitigated path from evidence to market compliance and commercial success for innovative products.

MEDICAL UMBRELLA
Scientific, clinical, medical communication, evidence, and commercial-medical functions
A. Medical Affairs & Scientific Strategy
Medical affairs strategy development
KOL mapping and engagement planning
Scientific communication strategy
Medical launch excellence
Medical information systems & query management
MSL training & capability building
Real-world evidence (RWE) program design
Evidence generation planning
Medical monitoring advisory support
B. Clinical Development & Drug Development Support
Pre-clinical strategy guidance
Clinical development plans & protocol design
Endpoint selection & comparator strategy
Risk-based monitoring strategy
Trial feasibility and site selection
Biostatistics & data management advisory
C. Medical Communication & Content Excellence
Medical writing (clinical, regulatory-linked, safety, HEOR)
Scientific content development
Promotional and non-promotional content
MLR review support (medical review component)
Publication planning & manuscript support
Digital scientific assets (MOA videos, e-learning, scientific platforms)
D. Market Access, Pricing & HEOR (Medical-Scientific Portion)
HEOR modelling (cost-effectiveness, budget impact)
Value story development & clinical narrative
AMCP/GVD value dossiers (scientific portion)
Clinical components for payer communication
E. Digital & AI (Medical-Focused Solutions)
AI-driven literature screening
AI-assisted medical writing
Digital clinical trial tools (ePRO, remote monitoring)
Data analytics dashboards for medical insights
REGULATORY UMBRELLA
Regulations, compliance audits, approvals, quality systems
A. Regulatory Affairs & Strategy
Regulatory pathway assessment (FDA, EMA, CDSCO, MHRA, PMDA)
Dossier preparation: IND, NDA, BLA, ANDA, CTA, variations
Labelling strategy, safety language, prescribing information
Regulatory intelligence (global updates monitoring)
Safety communication & REMS support
Regulatory strategy for product lifecycle
B. Pharmacovigilance
Periodic safety report support (DSUR, PBRER writing – regulatory component)
Safety governance structures
PV system audits & compliance checks
Signal detection framework advisory
LEGAL UMBRELLA
IP, compliance, ethics, contracts, and legal-risk domains
A. Intellectual Property (IPR) & Patent Strategy
Patent search & prior art evaluation
Freedom-to-operate (FTO) analysis
Patent drafting assistance
Lifecycle management (patent term strategies)
Brand protection and infringement risk assessment
B. Compliance, Ethics & Legal Governance
Ethics & compliance framework development
Anti-bribery, anti-corruption advisory
Data integrity compliance (legal risk component)
Contractual risk assessment
Policy development & SOP legal validation
C. Corporate & Strategic Legal Advisory
M&A due diligence (legal risk side)
Business contracts, licensing & partnership evaluations
IP clauses assessment in collaborations
Legal aspects of technology transfer agreements
D. Digital & AI (Legal/Safety Governance)
Data privacy compliance (GDPR, HIPAA equivalents)
Legal oversight of AI-based systems
Contractual risk in digital/AI vendor selection
We offer tailored IPR solutions for your unique needs, ensuring compliance and support in the Biopharmaceutical, MedTech and Healthcare sectors.
At LEELAWMED, we specialize in providing comprehensive Pharmaceutical Intellectual Property Rights (IPR) and Medicolegal consultancy services, with a focus on patent protection. With decades of expertise in the pharmaceutical sector, our team understands the complexities of patent law and its critical role in safeguarding innovations. We assist clients in obtaining, managing, and defending their patents, ensuring that their intellectual property remains protected in a highly competitive market.
Our services extend beyond patent filing; we also offer strategic guidance in navigating patent litigation, licensing agreements, and patent portfolio management. We work closely with pharmaceutical companies, university researchers, and healthcare providers to ensure that their discoveries are legally protected, allowing them to focus on innovation and growth.
With a deep understanding of the evolving regulatory environment, we provide tailored solutions that address both local and international patent challenges. Our medicolegal expertise helps clients navigate the intersection of law, ethics, and healthcare, offering risk mitigation strategies in the development and distribution of pharmaceutical products.
Trust “Leelawmed” to be your strategic partner in managing pharmaceutical / healthtec patents, safeguarding your innovations, and ensuring compliance with global standards.


Consulting Solutions
CONNECTING THE DOTS UNDER ONE NETWORK .....WITH EXPERTS FROM DIFFERENT DOMAINS.
We understand the dynamic needs of our clients, whether they are startups forging a growth path or established enterprises safeguarding their assets. With a steadfast focus on quality and efficiency, we are here to ensure that your business operates with the highest levels of integrity and compliance.
Medical, Legal & Regulatory Compliance
Leelawmed offers specialized Medical Affairs services tailored to the pharmaceutical, biotech, health-tech, and medical device sectors. Our multidisciplinary team ensures that your medical strategies align with regulatory standards and industry best practices.
Our offerings include:
Development and review of Patient Information Leaflets (PILs), Summaries of Product Characteristics (SmPCs), and Prescribing Information (PI).
Clinical evaluations and risk assessments to support product safety and efficacy.
Medical-Legal Reviews (MLR) for promotional materials, labelling, and clinical trial documents to ensure compliance with regulatory requirements.
Medical Writing Services
Our expert medical writers produce high-quality, evidence-based content to support both commercial and non-commercial needs:
Commercial Medical Writing: Creation of promotional materials, website content, and educational resources that effectively communicate product benefits while adhering to regulatory guidelines.
Non-Commercial Medical Writing: Development of scientific manuscripts, clinical study reports, and educational materials for healthcare professionals and patients.
Intellectual Property Rights (IPR) & Patent Services
Protecting your innovations is paramount. Leelawmed provides comprehensive IPR services to safeguard your intellectual assets:
Patentability assessments and strategic patent filing across multiple jurisdictions.
Trademark registration and protection to establish and maintain brand identity.
Copyright registration and content licensing to protect original works and manage usage rights.
Plagiarism detection and prevention strategies to maintain content integrity.
Trademark Information Services
Our trademark services are designed to help you navigate the complexities of brand protection:
Comprehensive trademark searches to identify potential conflicts.
Assistance with trademark application and registration processes.
Monitoring and enforcement services to protect against infringement.
At Leelawmed, we combine scientific expertise, legal precision, and regulatory insight to deliver tailored solutions that meet your unique needs.

Tailored biopharmaceutical, medicolegal, and healthcare solutions for domestic and international clients.
We don’t just provide services; we build relationships.
Our clients are our partners, and your success is our success.
Thank you for placing your trust in us. We look forward to supporting you in achieving your goals and advancing innovation in the healthcare and legal domains.
L E E L A W M E D
GLOBAL CONSULTING SOLUTIONS
Lifeline of Science & Technologies
Expertise in Bio-Pharmaceutical Research, Medical Affairs, Medical Communication, Publication, MLR, Regulatory, IPRs, Medicolegal, and Healthcare Consulting Solutions.
Contact Us:
For more information
e-mail: info@leelawmed.com
Phone: +91-8722860211
© 2026 | www.leelawmed.com | All rights reserved.

