Sponsor-CRO-Site-Integrated Solutions
Transforming Fragmented Clinical Operations into a Unified Development Platform
The pharmaceutical and clinical research ecosystem operates through three critical pillars — Sponsors (Pharmaceutical Companies), CROs, and Clinical Trial Sites (Hospitals & Academic Institutions). While each plays a vital role in drug development, operational silos, compliance variability, and misaligned execution models often lead to delays, regulatory setbacks, cost overruns, and data quality risks.
LEELAWMED Global Consulting Solutions delivers a structured integration that aligns Sponsors, CROs, and Trial Sites into a cohesive, compliant, and performance-driven ecosystem. Our approach ensures regulatory readiness, operational efficiency, scientific credibility, and accelerated development timelines.
We implement a structured integration platform connecting:
Pharmaceutical Sponsor ↔ CRO ↔ Clinical Trial Site
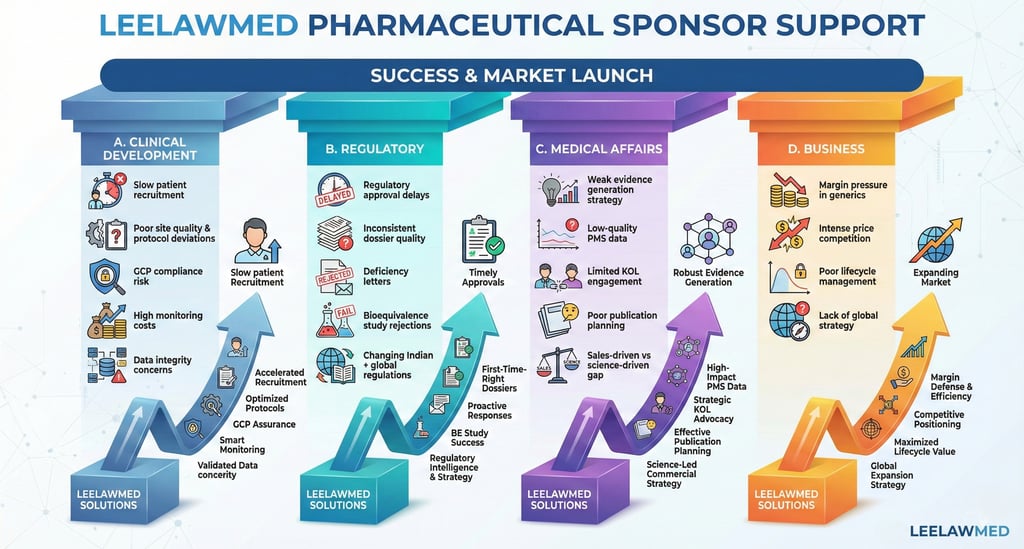
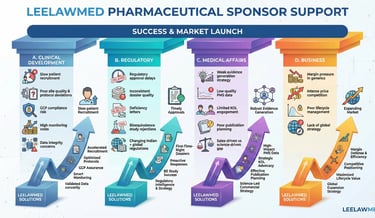
1) Pharmaceutical Sponsor Support
Strategic Clinical & Regulatory Partner for Sponsors
Pharmaceutical sponsors face mounting challenges, including regulatory scrutiny, rising development costs, competitive generics pressure, and global compliance expectations.
Our Sponsor-Focused Solutions:
Integrated Clinical Development Strategy
Protocol design optimization with feasibility validation
Statistical planning and endpoint alignment
Risk-based monitoring framework design
Real-world evidence strategy development
Regulatory Intelligence & Dossier Optimization
Global regulatory submission readiness
Bioequivalence regulatory alignment
Gap analysis and deficiency response strategy
Global harmonization (USFDA, EMA-aligned documentation planning)
Medical Affairs & Evidence Generation
Post-marketing surveillance (PMS) framework design
KOL engagement strategy
Publication planning and medical writing oversight
Lifecycle management advisory
Quality & Compliance Transformation
Audits & inspection readiness
SOP harmonization
TMF structuring & digital oversight
GCP compliance strengthening
Outcome for Sponsors:
Parallel and expertise guidance may result in reduced protocol amendments, faster approvals, improved data integrity, audit resilience, and optimized development costs.
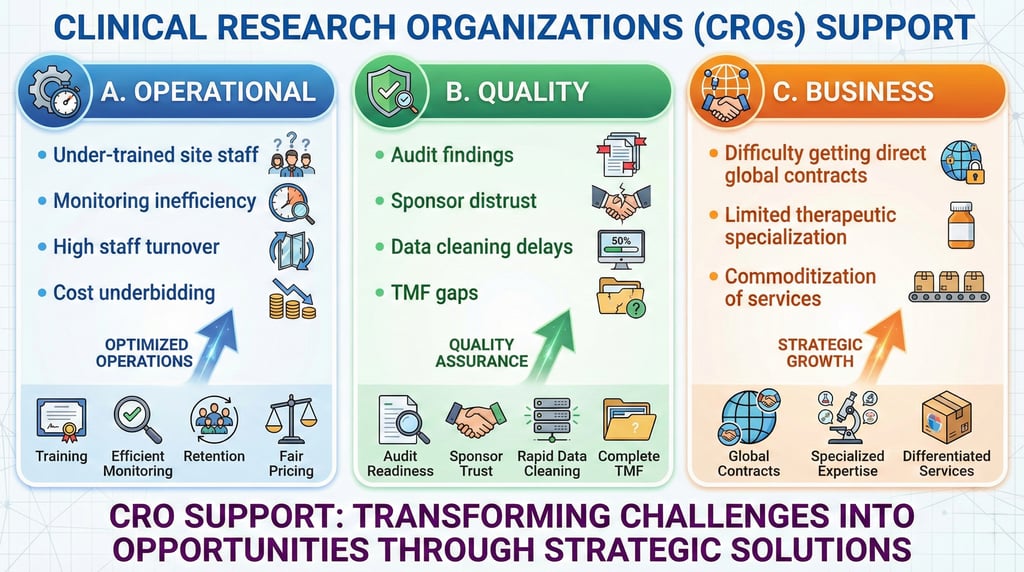
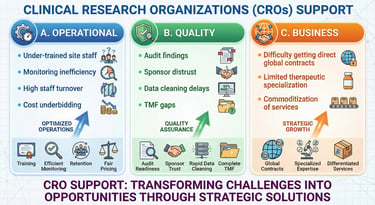
CRO Operational Excellence & Partnership Enablement
Enhancing CRO Capability, Quality & Global Readiness
LEELAWMED strengthens CRO infrastructure through structured quality and operational frameworks.
Our CRO Integration Solutions:
Operational Standardization
Harmonized SOP frameworks
Monitoring efficiency optimization
Site performance analytics
Feasibility validation
Quality Assurance & Audit Preparedness
GCP certification programs
TMF audit simulations
Documentation gap remediation
CAPA design & implementation
Specialized Scientific Support
Centralized biostatistics and study overview services
Medical & regulatory writing
Data cleaning & quality review
Business & Therapeutic Positioning
Therapeutic area specialization strategy
Capability enhancement programs
Sponsor confidence-building documentation
Outcome for CROs:
Improved sponsor confidence, stronger audit outcomes, enhanced operational efficiency, and sustainable revenue growth.
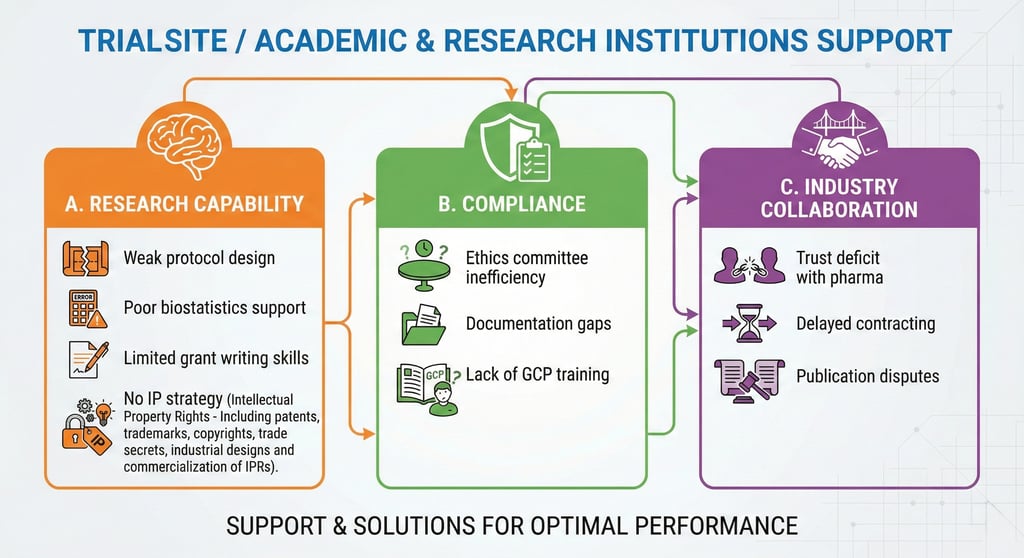
Clinical Trial Site (Hospital & Academic Institution) Strengthening
Building Research-Ready, Industry-Aligned Trial Sites
Hospitals and academic institutions possess strong clinical expertise but often lack structured regulatory, documentation, and translational research support.
Our Site Development Solutions:
Research Infrastructure Development
Clinical research SOP frameworks
Ethics committee process optimization
GCP awareness and assessment
Regulatory documentation systems
Translational Research & IP Structuring
Protocol development support
Biostatistics & methodological advisory
IP identification and commercialization pathway guidance
Industry collaboration facilitation
Performance & Governance Models
Study startup process acceleration
Budget negotiation advisory
Publication ethics and authorship structuring
Trial site certification programs
Outcome for Trial Sites:
Improved industry collaboration, increased study allocation, enhanced compliance, and stronger publication output.
L E E L A W M E D
GLOBAL CONSULTING SOLUTIONS
Lifeline of Science & Technologies
Expertise in Bio-Pharmaceutical Research, Medical Affairs, Medical Communication, Publication, MLR, Regulatory, IPRs, Medicolegal, and Healthcare Consulting Solutions.
Contact Us:
For more information
e-mail: info@leelawmed.com
Phone: +91-8722860211
© 2026 | www.leelawmed.com | All rights reserved.

